A 2D protein monolayer was fabricated by scientists by assembling lysozyme molecules — model protein in studying diseases like Amyloidosis. Amyloidosis is a rare disease that occurs when a protein called amyloid builds up in organs. This amyloid buildup can affect the working of organs like heart, kidneys, liver, spleen, nervous system and digestive tract. Lysozyme, a protein present in mucosal secretions and a principal component of airway fluid can be regarded as a model protein in studying diseases like Amyloidosis which ultimately leads to multi-organ dysfunction.
Scientists from the Institute of Advanced Study in Science and Technology, Guwahati (IASST), an autonomous institute of North-East India under the Department of Science and Technology (DST) have assembled lysozyme molecules as a 2D monolayer at the interface of pure aqueous subphase. The research group, led by Dr. Sarathi Kundu, Associate Professor, along with Himadri Nath, a junior Research Fellow used the 2D protein monolayer to understand the behaviour of lysozyme molecules at air-water as well as at air-solid interface with the help of a technique called Langmuir-Blodgett (LB) technique.
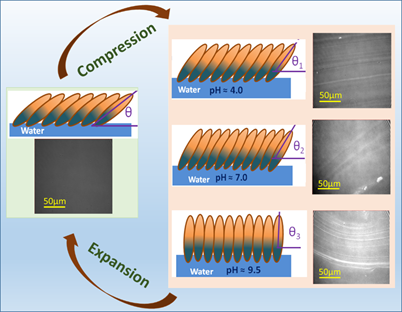
The physical properties of lysozyme molecules at air-water interface were investigated under the variation of surface pressure and subphase pH conditions in the study recently published in the RSC Advances under the reputed RSC publishers. The compressible behaviour of lysozyme monolayers were correlated to the stripe-like domains formed with increase in surface pressure.
Lysozyme molecules at the air-water interface and their structural or conformational changes in variable pH conditions can be considered as a model system to study Amyloidosis disease, which occurs because of the misfolding and agglomeration of lysozyme molecules.




