
Author: Eve Pope, Technology Analyst at IDTechEx
The chemicals industry depends heavily on finite fossil fuel feedstocks and is responsible for 2% of global anthropogenic CO2 emissions. A new report from IDTechEx, “Carbon Dioxide Utilization 2025-2045: Technologies, Market Forecasts, and Players”, explores how captured CO2 could be utilized as a feedstock for hundreds of different chemicals instead, providing an economic and environmental incentive to capture carbon dioxide and create a circular economy.
Why is CO2 utilization in chemical production important?
Carbon capture is viewed as an essential technology for lowering global carbon dioxide emissions as it can decarbonize hard-to-abate sectors. However, carbon capture technologies are expensive. If captured carbon can be utilized to make profitable chemical products, this revenue stream can provide an economic incentive to accelerate the uptake of CCUS (carbon capture, utilization, and storage) technologies until stronger legislation that promotes CO2 storage emerges.
While many CO2-derived chemicals do not represent net-negative or net-zero products, they do still represent reductions in emissions compared to the fossil fuel-based status quo. They should not be overlooked as a decarbonization tool.
How are chemicals made from CO2?
Millions of different compounds contain carbon. Examples include fuels such as gasoline and methanol, as well as everyday plastics. Recycling captured CO2 and combining it with low-carbon hydrogen provides a new route to these carbon-containing chemicals. Because carbon dioxide is a thermodynamically stable molecule, an energy input is needed for reactions to occur. Currently, the most mature synthesis pathways for CO2-derived chemicals are thermocatalytic – where heat and catalysts yield favorable reaction kinetics.
However, there is considerable interest in synthetic routes that can be performed at ambient temperatures and pressures, with reduced energy demand and cost. Alternative synthesis pathways championed by start-ups include biological conversion and electrochemical approaches.
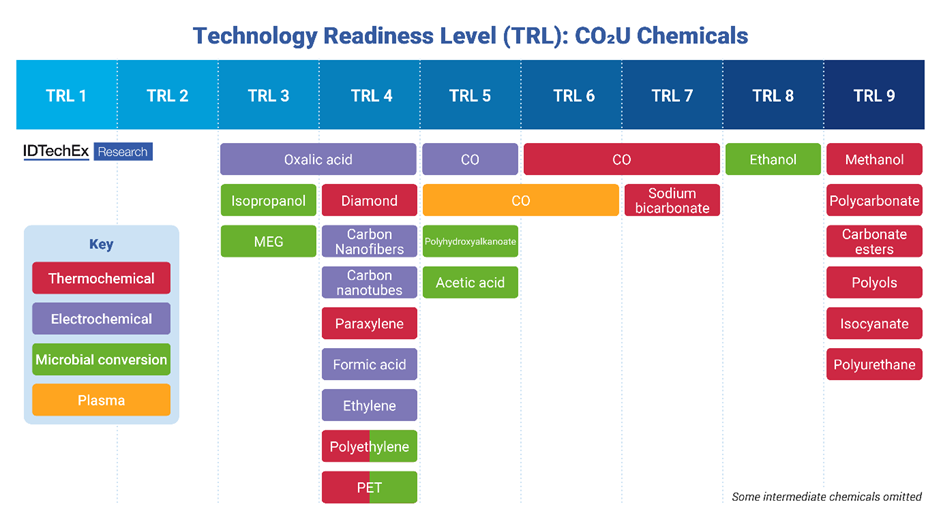
Technology readiness level (TRL) for various CO2 utilization chemicals made via different synthesis routes. IDTechEx defines CO2 utilization TRL based on suitability for utilizing large volumes of carbon dioxide. Source: IDTechEx
Biological conversion of CO2
Nature is already an expert at CO2 utilization. During photosynthesis, plants convert carbon dioxide into glucose. Several start-ups are seeking to harness the power of photosynthesis using genetically engineered microalgae or cyanobacteria to produce fuels and chemicals. A notable example is Cemvita. Cemvita produces a C16-C18 oil with a similar profile to palm oil that shows promise for sustainable aviation fuel.
However, scaling large light-exposing surfaces where reactions can take place is not straightforward, leading many players to favor chemosynthesis over photosynthesis. In chemosynthetic biological conversion, inorganic molecules such as H2 provide a source of energy instead of light. LanzaTech – already producing over 100,000 tonnes per annum of ethanol from captured CO2 – is commercializing this approach using acetogenic bacteria.
Bioreactors are also often difficult to scale up. For biological conversion pathways in CO2 utilization, a specific technical challenge reported by several players involves limited gas-liquid mass transfer of the CO2 gas within the reactor. LanzaTech’s innovation in this space combined a novel device for microbubble formation with a reactor geometry and liquid circulation approach that increased gas mass transfer coefficients. Another solution, being developed by earlier stage player CarbonBridge, is direct gas fermentation, which avoids using a liquid media.
Electrochemical conversion of CO2
For electrochemical conversion of CO2, the required inputs are water and captured carbon dioxide. Low-temperature CO2 electrolysis can generate syngas and multi-carbon products such as ethylene, ethanol, and propanol. Sustainable chemicals producer Avantium, an innovator in this space, has started demonstrating the production of CO2-derived formic acid and oxalic acid using its Volta Technology platform. In the realm of e-fuels, Twelve’s commercial Moses Lake SAF project opened its doors in 2024. This facility uses a low-temperature proton exchange membrane CO2 electrolyzer to first produce syngas, then well-established thermocatalytic Fischer-Tropsch synthesis produces the longer hydrocarbon chains of the e-fuel.
High-temperature CO2 electrolysis can also be performed if solid oxide electrolyzers are used. The high-temperature operation improves the kinetics of H2O and CO2 electrolysis and the catalytic activity compared to low-temperature electrolyzers, reducing the need for expensive catalysts. However, high-temperature operation causes accelerated degradation and increases maintenance requirements.
Thermocatalysis generally demands large-scale production to be cost-effective. In contrast, electrochemical methods could, in theory, be operated economically at smaller scales. This could lead to more distributed chemical manufacturing, reducing transportation costs and giving greater supply chain control.
Outlook
Chemicals are already being made by recycling captured CO2 at a large scale. Players are now focused on extending production to new CO2-derived chemicals and alternative synthesis pathways. Biological conversion and electrochemistry for CO2 utilization show particular promise, although these routes may need to be combined with additional thermocatalytic steps to achieve the most economical synthesis. By 2045, IDTechEx forecasts 19 million tonnes per annum of drop-in chemicals will be synthesized from captured CO2.
To find out more about IDTechEx’s “Carbon Dioxide Utilization 2025-2045: Technologies, Market Forecasts, and Players” report, including downloadable sample pages, please visit www.IDTechEx.com/CO2U.
For the full portfolio of energy and decarbonization market research available from IDTechEx, please see www.IDTechEx.com/Research/Energy.
Upcoming free-to-attend webinar
How Profitable, Emerging CO2 Utilization Applications Can Accelerate CCUS Deployment
Eve Pope, Technology Analyst at IDTechEx and author of this article, will be presenting a free-to-attend webinar on the topic on Tuesday 1 October 2024 – How Profitable, Emerging CO2 Utilization Applications Can Accelerate CCUS Deployment.
This webinar will reveal insights into CO2U technologies, and its content includes:
- Overview of CO2 utilization pathways and products
- Discussion of scale, TRL (technology readiness level), and profitability for promising CO2U technologies
- Analysis of additional revenue streams from CO2 utilization applications – including waste disposal fees, carbon credits, and 45Q tax credits
- Key considerations for CO2 utilization market growth
We will be holding exactly the same webinar three times in one day. Please click here to register for the session most convenient for you.
If you are unable to make the date, please register anyway to receive the links to the on-demand recording (available for a limited time) and webinar slides as soon as they are available.
IDTechEx Hydrogen & CCUS Technology Briefing
Tuesday 22nd October 2024: Hamburg, Germany
IDTechEx is offering the opportunity to learn more about the future of the CCUS and hydrogen markets directly from industry experts at the upcoming IDTechEx Hydrogen & CCUS Technology Briefing in Hamburg, Germany on October 22nd 2024. The briefing is free to attend but spaces are limited – please contact research@IDTechEx.com




